Abstract
Background
Despite recent advances in treatment options, multiple myeloma (MM) remains incurable. We previously presented preliminary data from an ongoing Phase 1/2 trial (NCT03761108) demonstrating that REGN5458 (a BCMAxCD3 bispecific antibody) monotherapy had an acceptable safety and tolerability profile with early, deep, and durable responses in heavily pretreated patients (pts), with at least triple-refractory RRMM, (Madduri, ASH 2020, O291). Here we describe updated safety, overall response, and response durability in pts treated in the Phase 1 portion of this study.
Methods
The primary objectives of the Phase 1 portion of the study are to assess the safety, tolerability, and occurrence of dose-limiting toxicities of REGN5458 and to determine a recommended Phase 2 dose regimen (RP2DR). Key secondary objectives include: assessment of objective response rate as determined by the investigator, duration of response (DOR), and minimal residual disease status; pharmacokinetic (PK) evaluation; and characterization of immunogenicity. Pts with progressive RRMM, who were triple-refractory, or intolerant to prior lines of systemic therapy including a proteasome inhibitor, immunomodulatory agent, and anti-CD38 antibody are treated with REGN5458 monotherapy following a modified 3+3 dose-escalation design (4+3). Treatment consists of 16 weekly infusions of REGN5458, followed by q2w dosing, until disease progression. Response assessments are measured using modified International Myeloma Working Group criteria.
Results
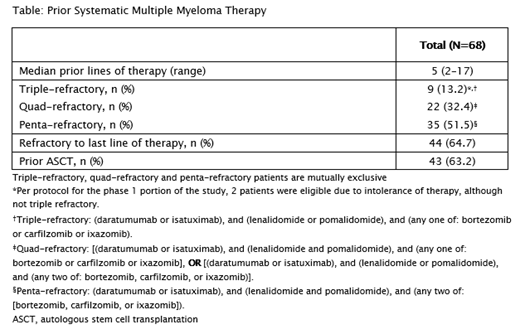
As of data cut-off (June 10, 2021), 68 pts were treated with REGN5458 in the dose escalation cohort with full doses ranging from 3-400 mg. The median age at enrollment was 64 years (range, 41‒81) and 20.6% pts were ≥75 years. As per Revised International Staging System, stage was 1, 2 or 3 in 14.7%, 60.3% and 23.5% of pts respectively. Pts had a median of 5 prior lines of systemic therapy (range, 2-17) with the majority of pts (51.5%) being penta-refractory (see Table). The median duration of follow-up was 2.4 months (range, 0.1-20.8).
Treatment-emergent adverse events (TEAE) were reported in 66 pts (97.1%), and Grade (Gr) ≥3 TEAEs in 52 (76.5%) pts. The most frequent TEAEs were fatigue in 29 pts (42.6%), Gr 1/2 in 26 pts (38.2%), Gr 3 in 3 pts (4.4%); cytokine release syndrome (CRS) in 26 pts (38.2%), CRS was Gr 1 in 23 pts (33.8%) and Gr 2 in 3 pts (4.4%). No pt had Gr ≥3 CRS or discontinued treatment due to CRS. There were no Gr ≥3 neurotoxicity events. Nausea was reported in 22 pts (32.4%). The severity of nausea was Gr 1 in 23.5% of pts and Gr 2 in 8.8% of pts.
Treatment-related adverse events (TRAE) were reported in 56 patients (82.4%). The most frequent hematologic TRAE was neutropenia in 11 pts (16.2%), with Gr ≥3 severity in 9 of these pts (13.2%). The most frequent non-hematologic TRAEs were CRS (38.2%) and fatigue (20.6%). The safety profile was consistent across all dose levels, and there was no correlation between CRS and the full dose of REGN5458.
Responses were observed at all dose levels. Amongst pts treated at the 96 and 200 mg dose levels, the response rate was 73.3% (11/15). Across all dose levels, 92.6% (n=25) of all responders achieved at least a very good partial response and 48.1% (n=13) of responders had a complete response (CR) or stringent CR. Pts without extramedullary plasmacytomas (EMP) responded more frequently than those with EMP. The Kaplan-Meier estimated median DOR was not reached and the probability of DOR ≥8 months was 92.1% (95% confidence interval: 72.1, 98.0), with responses ongoing up to 19 months at the latest data cut-off.
Disease response was not impacted by level of BCMA expression in the core biopsy, as assessed by immunohistochemistry.
Additional PK and biomarker data will be available at the time of presentation. Updated safety and efficacy data will also be presented.
Conclusions
In this updated analysis of the first-in-human study, REGN5458 continues to show an acceptable safety and tolerability profile, with Gr 2 CRS in only 4.4% of patients, and no Gr ≥3 CRS or neurotoxicity events. No new safety signals were observed during the additional follow-up period. Early, deep, and durable responses were seen in triple- to penta-refractory patients with RRMM, with a 73.3.% response rate at the combined 96 and 200 mg dose levels. The Phase 2 portion of the study is currently recruiting.
Zonder: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; Alnylam: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Caelum Biosciences: Consultancy; Intellia: Consultancy; Regeneron: Consultancy. Richter: BMS, Karyopharm, Antengene: Membership on an entity's Board of Directors or advisory committees; Adaptive biotechnologies: Speakers Bureau; Janssen, Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Tisch Cancer Institute: Icahn School of Medicine at Mount Sinai: Current Employment. Bumma: Amgen, Sanofi: Speakers Bureau; Janssen, Oncopeptides, Sanofi: Consultancy. Hoffman: BMS, Celgene: Honoraria. Bensinger: BMS, Janssen, Poseida, Regeneron, Trillium: Research Funding; Amgen, BMS, Janssen, Sanofi: Speakers Bureau. Xu: Regeneron Pharmaceuticals, Inc: Current Employment; Regeneron Pharmaceuticals, Inc: Current holder of stock options in a privately-held company. Chokshi: Regeneron Pharmaceuticals, Inc: Current holder of stock options in a privately-held company; Regeneron Pharmaceuticals, Inc: Current Employment. Boyapati: Regeneron Pharmaceuticals, Inc: Current Employment; Regeneron Pharmaceuticals, Inc: Current holder of stock options in a privately-held company. Sharma: Regeneron Pharmaceuticals, Inc.: Patents & Royalties: US17/112,564: Methods of Treating Multiple Myeloma with Bispecific anti-BCMA X anti-CD3 Antibodies (Status: Pending). Rodriguez Lorenc: Regeneron Pharmaceuticals, Inc: Current Employment; Regeneron Pharmaceuticals, Inc, Novartis Pharmaceuticals: Current holder of stock options in a privately-held company. Kroog: Regeneron Pharmaceuticals, Inc: Current Employment; Regeneron Pharmaceuticals, Inc: Current holder of stock options in a privately-held company. Lentzsch: Magenta Therapeutics: Current equity holder in publicly-traded company; Ossium Health: Consultancy; Caelum Biosciences: Consultancy, Current holder of individual stocks in a privately-held company; Oncopeptides: Consultancy; Sanofi: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Takeda: Consultancy; GSK: Consultancy; AbbVie: Consultancy; Celularity: Consultancy; Janssen: Consultancy; Kadmon: Current equity holder in publicly-traded company. Jagannath: Janssen Pharmaceuticals: Consultancy; Takeda: Consultancy; Legend Biotech: Consultancy; Karyopharm Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy; Sanofi: Consultancy.
The data described in the abstract will report on use of REGN5458 in a Phase 1 clinical trial of patients with relapsed/refractory multiple myeloma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal